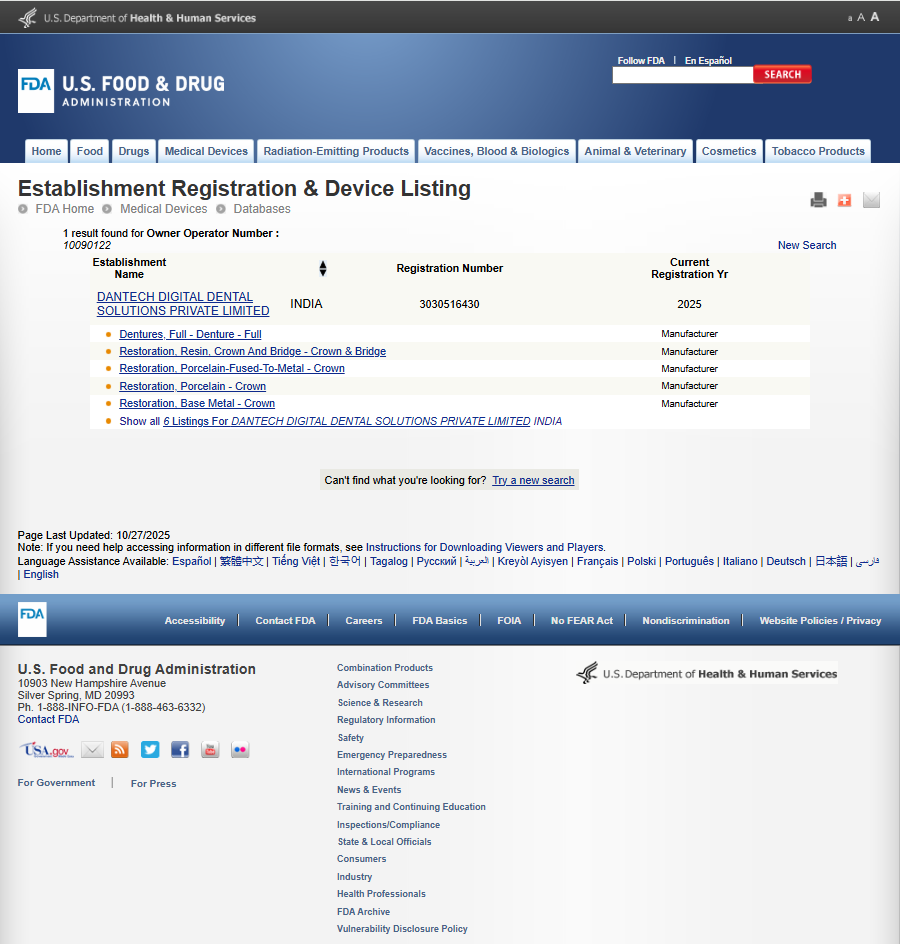
Dantech Dental Lab: Certified for Global Quality and Compliance
At Dantech Dental Lab, quality is at the heart of everything we do. We are proud to be an ISO 13485:2016 certified, UK-MHRA registered, and U.S. FDA registered dental laboratory — a recognition of our commitment to global standards in safety, quality, and regulatory compliance.
The ISO 13485:2016 certification demonstrates our adherence to internationally recognized quality management systems specific to medical devices. It ensures that every stage of our process—from design and material selection to manufacturing and delivery—meets the highest levels of consistency, traceability, and safety.
Our UK-MHRA registration confirms our conformity with the United Kingdom’s regulatory framework for medical devices, while our FDA registration highlights our compliance with the United States Food and Drug Administration’s stringent standards. Together, these accreditations reinforce Dantech’s position as a trusted global partner for advanced dental solutions.
Every product manufactured at Dantech undergoes rigorous quality assurance protocols, ensuring precision, durability, and clinical reliability. These certifications reflect our unwavering commitment to innovation, continuous improvement, and excellence in digital dentistry.
At Dantech Dental Lab, we don’t just meet industry standards—we set them. Our certifications are a testament to our promise of delivering world-class dental restorations that combine technology, trust, and craftsmanship to achieve superior patient outcomes worldwide.